In the world of in vitro diagnostics (IVD), product variety ranges from conventional reagents and kits, instruments up to software. Our interdisciplinary team is able to provide you competent support throughout development and certification of your product, no matter whether it is a conventional ELISA test, stand-alone software, diagnostic services, high-risk list A product or a point-of-care test system.
Our aim is to keep up with the latest technological and regulatory trends to provide you with customized and pragmatic solutions: Definition of certifiable product components, requirements engineering, designing the analytical and clinical performance evaluation, implementation of CTS requirements, risk management or usability engineering – we got you covered!
On May 25th 2017, the new European IVD Regulation (IVDR) has entered into force with a transition period of 5 years. One of the major changes is the introduction of a new classification system.
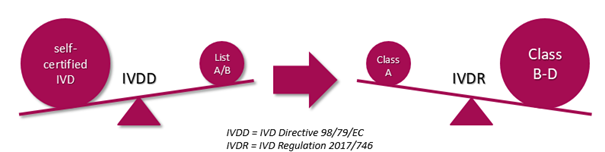
Following this new system, a much higher portion of products will fall under supervision of a Notified Body during the conformity assessment procedure. Learn more about how we can assist you here or get in contact with us!
Our experience includes: